ARTICLE
ARTICLE
Seeking the Source of Ebola
Seeking the Source of Ebola
The latest Ebola crisis may yield clues about where it hides between outbreaks.
Grades
9 - 12
Subjects
Biology, Health
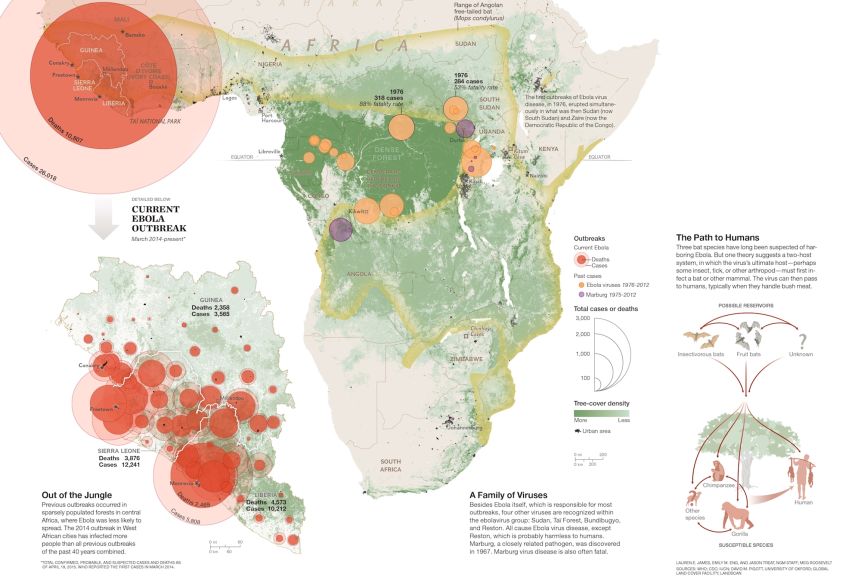
Image
Ebola Map
A map of the 2014-2015 Ebola outbreak and how it is believed to have spread throughout Africa.
Graphic by James Lauren E.
No one foresaw, back in December of 2013, that the little boy who fell ill in a village called Méliandou, in Guinea, West Africa, would be the starting point of a gruesome epidemic, one that would devastate three countries and provoke concern, fear, and argument around the planet.
No one imagined that this child’s death, after just a few days’ suffering, would be only the first of many thousands. His name was Emile Ouamouno. His symptoms were stark—intense fever, black stool, vomiting—but those could have been signs of other diseases, including malaria. Sad to say, children die of unidentified fevers and diarrheal ailments all too frequently in African villages. But soon the boy’s sister was dead too, and then his mother, his grandmother, a village midwife, and a nurse. The contagion spread through Méliandou to other villages of southern Guinea. This was almost three months before the word “Ebola” began to flicker luridly in email traffic between Guinea and the wider world.
The public health authorities based in Guinea’s capital, Conakry, and the viral disease trackers from abroad weren’t in Méliandou when Emile Ouamouno died. Had they been, and had they understood that he was the first case in an outbreak of Ebola virus disease, they might have directed some timely attention to an important unknown: How did this boy get sick? What did he do, what did he touch, what did he eat? If Ebola virus was in his body, where did it come from?
Among the most puzzling aspects of Ebola virus, since its first recognized emergence almost four decades ago, is that it disappears for years at a time. Since a 1976 outbreak in what then was Zaire (now the Democratic Republic of the Congo) and a simultaneous episode with a closely related virus in what was then southern Sudan (now South Sudan), the sequence of Ebola events, large and small, has been sporadic. During one stretch of 17 years (1977-1994) not a single confirmed human death from infection with Ebola virus occurred. This is not a subtle bug that simmers delicately among people, causing nothing more than mild headaches and sniffles. If it had been circulating in human populations for those 17 years, we would have known.
A virus can’t survive for long, or replicate at all, except within a living creature. That means it needs a host—at least one kind of animal, or plant, or fungus, or microbe, whose body serves as its primary environment and whose cell machinery it can co-opt for reproducing. Some harmful viruses abide in nonhuman animals and only occasionally spill into people. They cause diseases that scientists label zoonoses. Ebola is a zoonosis, an especially nasty and perplexing one—killing many of its human victims in a matter of days, pushing others to the brink of death, and then vanishing. Where does it hide, quiet and inconspicuous, between outbreaks?
Not in chimpanzees or gorillas; field studies have shown that Ebola often kills them too. Dramatic die-offs of chimps and gorillas have occurred around the same time and in the same area as Ebola virus disease outbreaks in humans, and some carcasses have tested positive for signs of the virus. Scavenging ape carcasses for food, in fact, has been one of the routes by which humans have infected themselves with Ebola. So the African apes are highly unlikely to harbor Ebola. It hits them and explodes. It must lurk somewhere else.
The creature in which a zoonotic virus exists over the long term, usually without causing symptoms, is known as a reservoir host. Monkeys serve as reservoir hosts for the yellow fever virus. Asian fruit bats of the genus Pteropus are reservoirs of Nipah virus, which killed more than a hundred people during a 1998-99 outbreak in Malaysia. Fruit bats also host Hendra virus in Australia, where it drops from bats into horses, with devastating effect, and then into horse handlers and veterinarians, often killing them. The passage event, when a virus goes from its reservoir host to another kind of creature, is termed spillover.
As for the reservoir host of Ebola—if you have heard that fruit bats again are the answer, you’ve heard supposition misrepresented as fact. Despite arduous efforts by some intrepid scientists, Ebola virus has never been tracked to its source in the wild.
“Where is it when it’s not infecting humans?” Karl M. Johnson said to me recently. Johnson is an eminent virologist, a pioneer in Ebola research, the former head of the Viral Special Pathogens Branch at the Centers for Disease Control and Prevention (CDC). He led the international response team against that initial 1976 outbreak in Zaire, a harrowing venture into the unknown. He also led a team that isolated the virus in a CDC lab, demonstrated that it was new to science, and named it after a modest Zairean waterway, the Ebola River. Johnson wondered back then about its hiding place in the wild. But the urgency of human needs during any Ebola outbreak makes investigations in viral ecology difficult and unpopular. If you’re an African villager, you don’t want to see foreigners in moon suits methodically dissecting small mammals when your loved ones are being hauled away in body bags. Thirty-nine years later, although we’re beginning to learn a bit, Johnson said, the identity of the reservoir host “is still largely a monster question mark out there.”
A Rain of Bats
In April 2014, soon after word spread that the cluster of deaths in southern Guinea involved Ebola, Fabian Leendertz arrived there with a team of researchers. Leendertz is a German disease ecologist and veterinarian, based at the Robert Koch Institute in Berlin, who studies lethal zoonoses in wildlife, with special attention to West Africa. He reached southern Guinea by driving overland from Ivory Coast, where he has worked for 15 years in Taï National Park on disease outbreaks among chimpanzees and other animals. He brought with him three big vehicles, full of equipment and people, and two questions. Had there been a recent die-off among chimps or other wildlife, possibly putting meat-hungry humans at risk from infected carcasses? Alternatively, had there been direct transmission from the Ebola reservoir host, whatever it was, into the first human victim? Leendertz knew nothing at that point about Emile Ouamouno. His team spoke with officials and local people and walked survey transects through two forest reserves, finding neither testimony nor physical evidence of any remarkable deaths among chimpanzees or other large mammals. Then they shifted their attention to the village of Méliandou, talked with people there, and heard a very interesting story about a hollow tree full of bats.
These were small bats, the quick-flying kind that echolocate and feed on insects, not the big creatures that fly out majestically at dusk, like a Halloween vision of nocturnal crows, to eat fruit. The locals called them lolibelo. They were dainty as mice and smelly, with wriggly tails that extended beyond their hind membranes. Showing pictures and taking descriptions, Leendertz’s team ascertained that the villagers were probably talking about the Angolan free-tailed bat (Mops condylurus). These bats had roosted in great numbers within a big, hollow tree that stood beside a trail near the village. Then, just weeks before, the tree had been burned, possibly during an attempt to gather honey. From the burning tree came what the people remembered as “a rain of bats.” The dead bats were gathered up, filling a half dozen hundred-pound rice sacks, and might have been eaten except for a sudden announcement from the government that because of Ebola, consuming bush meat was now prohibited. So the Méliandou villagers threw the dead bats away.
And there was something else about that hollow tree, the villagers told Leendertz’s team. Children, possibly including Emile Ouamouno, used to play in it, sometimes catching the bats. They would even roast them on sticks and eat them.
Leendertz consulted a colleague with expertise in recovering DNA from environmental samples, who told him it might be feasible to find enough beneath the tree to identify the bat species that had roosted there. “So I started running around with my tubes and spoon collecting soil,” Leendertz told me. Back in Berlin, genetic sequencing confirmed the presence of Angolan free-tailed bats. So this creature—an insectivorous bat, not a fruit bat—joined the list of candidates for the role of Ebola’s reservoir host.
The Hitchhiker
The first clues in this long mystery—clues that seemed to point toward bats—arose from disease outbreaks caused by Marburg virus, Ebola’s slightly less notorious relative within the group known as filoviruses. The story of Ebola is closely connected with that of Marburg, according to a seasoned South African virologist named Robert Swanepoel, who has long studied them both.
“The two are interlinked,” he said, as we sat before a computer screen in his Pretoria home, looking at photographs from his archive. Swanepoel, who hides a genial heart within a bearish exterior, is retired from the National Institute for Communicable Diseases (NICD), in Johannesburg, where he ran the Special Pathogens Unit for 24 years, but is still busy with research and bristling with ideas and memories.
Back in 1967, nine years before Ebola itself was recognized, a shipment of Ugandan monkeys intended for medical research arrived in Frankfurt and Marburg, in West Germany, and Belgrade, in Yugoslavia, bringing with them an unknown but dangerous virus. Laboratory workers became infected in each place, and then, secondarily, some family members and health workers. Among 32 confirmed cases, seven people died. The new virus, a spooky, filamentous thing, like a strand of toxic vermicelli, was given the name Marburg virus. Eight years later an Australian student died of Marburg virus disease in a Johannesburg hospital after a hitchhiking trip across Rhodesia (now Zimbabwe). He and his girlfriend—she got sick too but recovered—had done a few things that might have exposed them to infection: slept on the ground in a pasture, bought some raw eland meat, fed some caged monkeys. And they had visited the Chinhoyi Caves, a complex of caverns and sinkholes in northern Rhodesia that, like many caves in Africa, have been known to harbor bats. Along the way the hitchhiker also sustained some sort of insect or spider bite, which raised a painful red welt on his back. Investigation of his case in the immediate aftermath focused much on the bite, little on the caves.
Two other early cases of Marburg virus disease did cast some suspicion on caves and the bats that roost within them. In 1980 a French engineer who worked at a sugar factory near the base of Mount Elgon, in western Kenya, ventured into Kitum Cave, a deep passage into the volcanic rock of the mountain sometimes entered by elephants looking for salt. The engineer’s cave visit was evidently a bad idea; he died of Marburg in a Nairobi hospital. In 1987 a Danish schoolboy climbed the mountain and explored the same cave during a family vacation, and he died of an infection with a virus (now known as Ravn virus) closely related to Marburg. These events engaged the notice of Swanepoel, down in Johannesburg. In 1995 came another outbreak—Ebola this time, not Marburg—centered on the city of Kikwit in what is now the Democratic Republic of the Congo (DRC). The chain of human-to-human infections, which totaled 315 cases and 254 deaths, began with a man who farmed manioc and made charcoal in a forest area at the city’s edge. Swanepoel flew to Kikwit, joining an international team of responders. He came down with malaria, went home, recovered, and in early 1996, with the support of the World Health Organization, returned. His primary task was to look for the reservoir host, searching the same ecosystem where the outbreak had begun at the same time of year. “Already by that stage,” he told me, “bats were on my mind.”
Swanepoel and his crew at Kikwit took blood and tissue not only from bats but also from a wide selection of other animals, including many insects. Screening those samples back at his lab in Johannesburg, he found no evidence of Ebola. So he tried an experimental approach, one that seemed almost maniacally thorough. Working in NICD’s high-containment suite—biosafety level 4 (BSL-4), the highest—he personally injected live Ebola virus from the Kikwit outbreak into 24 kinds of plants and 19 kinds of animals, ranging from spiders and millipedes to lizards, birds, mice, and bats, and then monitored their condition over time. Though Ebola failed to take hold in most of the organisms, a low level of the virus—which had survived but probably hadn’t replicated—was detected in a single spider, and bats sustained Ebola virus infection for at least 12 days. One of those bats was a fruit bat. Another was an Angolan free-tailed bat, the same little insectivore that would later catch Fabian Leendertz’s attention in Méliandou. It was proof of principle, though not of fact: These creatures could be reservoir hosts.
Ten Thousand Haystacks
The events in Kikwit highlighted an important difference between Marburg and Ebola viruses that has persisted: Whereas outbreaks of Marburg virus disease usually begin around caves and mines, Ebola virus disease outbreaks usually begin with hunting and carcass scavenging, which are forest activities. This suggests the two viruses may emerge from two different kinds of reservoir hosts—or if bats are the hosts, two different kinds of bats, cave roosters and tree roosters.
The pattern was reaffirmed during a cluster of Marburg outbreaks from 1998 to 2000, centered on a derelict gold-mining town called Durba, in the DRC. Bob Swanepoel led another expedition and found multiple chains of infection, most or all of which started with miners who worked underground. Miners who worked at open pits in the daylight were far more likely to stay healthy. This led Swanepoel to suspect cave-roosting Egyptian fruit bats as the virus source, though he didn’t publish his suspicion at the time.
Then, beginning in late 2001 and extending into 2003, another series of small, independent outbreaks—of Ebola again, not Marburg—afflicted villagers in the densely forested borderlands of Gabon and the Republic of the Congo (which are west of the DRC, on the other side of the Congo River). Roughly 300 people became infected; almost 80 percent died. Meanwhile gorillas, chimpanzees, and duikers, small forest antelopes, started turning up dead in the same region. Each human outbreak seemed to start with an unfortunate person, usually a hunter, who’d handled an animal carcass.
“People were dying, and different animals were dying,” said Janusz Paweska, nowadays Swanepoel’s successor as head of Special Pathogens at NICD, when I visited him in Johannesburg. “So we thought, This is a good time to hunt for the Ebola reservoir.”
Swanepoel enlisted Paweska and others, then arranged a partnered expedition with Eric Leroy, a French virologist based in Gabon who had responded to earlier Ebola outbreaks there. He met with Leroy in Gabon’s capital, Libreville, before heading into the field.
“I gave him a long story about how historically bats have been involved in Ebola and Marburg,” Swanepoel told me. His team, he informed Leroy, had found fragments of Marburg, for instance, in the underground bats at Durba. Swanepoel had brought rodent traps, mist nets, and other collecting gear to Gabon. “Although I was fixated on bats, I said we had to cover everything,” he recalled. That would include a variety of mammals, birds, mosquitoes, biting midges, and other insects. Swanepoel’s group took home a third of the specimens and sent a third to the CDC in Atlanta, leaving a third to be tested by Leroy. The processing moved slowly in Swanepoel’s lab and at the CDC, amid many other projects, and yielded no positives. “We drew a blank.”
But Leroy’s group went back. Eventually his team made three field trips to the border area, capturing and sampling more than a thousand animals, including 679 bats, on which Leroy too was now fixated. In 16 of those bats, belonging to three different fruit-eating species, they found antibodies—proteins marshaled by the immune system—that had reacted against Ebola virus. In 13 other fruit bats they detected very short fragments of Ebola RNA. It’s important to note that those two kinds of evidence, antibodies and viral fragments, are analogous to finding the footprints of a Yeti in snow. You might or might not have something real. Isolating live virus—that is, growing fresh and infectious Ebola from a tissue sample—is the higher standard of evidence, almost like finding a real Yeti’s foot attached to a real Yeti in a leghold trap. Leroy’s group didn’t succeed in growing live virus from any samples. Still, in 2005 the journal Nature published a paper on these results, written by Leroy but with Swanepoel and Paweska credited as co-authors, titled “Fruit Bats as Reservoirs of Ebola Virus.” That paper, though cautious and provisional, is the primary source for all those careless, overly certain assertions you’ve seen in the popular media during the past year to the effect that Ebola virus resides in fruit bats.
Possibly it does. Or not. The paper itself says maybe.
“You tried to isolate live virus?” I asked Leroy during my stop in Gabon. He’s a courteous, dapper Frenchman, now director of the Centre International de Recherches Médicales de Franceville, who works in a white shirt and dark tie, at least when he’s not wearing a full protective suit in his BSL-4 lab or Tyvek coveralls in the forest. “Yes. Many, many, many times trying to isolate the virus,” he said. “But I never could. Because it was—the viral load was very, very low.” Viral load is the quantity of virus in the solid tissues or blood of the creature, and it tends to be much lower in a reservoir host than in an animal or person suffering an acute infection.
That’s just one of three reasons why finding a reservoir host is difficult, Leroy explained. The second is that, in addition to low viral load within each animal, the virus may exist at low prevalence within a population. Prevalence is the percentage of positive individuals at a given time, and if that happens to be as little as one animal in a hundred, then “the probability to detect and to catch this infected animal is very low.” If a single kind of animal amid the great diversity of tropical forests represents a needle in a haystack, then one infected individual within one population of animals amid such diversity represents one needle in ten thousand haystacks.
And the third constraint on the search for a reservoir host? “It’s extremely expensive,” Leroy said.
The Perfect Holiday
The cost of field operations in remote forest locations, as well as the competing demands upon institutional resources, has hindered even veteran researchers such as Swanepoel and Leroy from mounting long-term, continuous studies of the Ebola reservoir question. Instead there have been short expeditions, organized quickly during an outbreak or just as a crisis was ending. But going to the site of a human outbreak to do research on the ecology of the virus is logistically nightmarish and, as I’ve mentioned, offensive to local people. So those expeditions get delayed. The problem with delay is that the prevalence of Ebola virus within its host population, the viral load within individual hosts, and the abundance of virus being shed into the environment may all fluctuate seasonally. Miss the right season, and you might miss the virus.
Fabian Leendertz tried to address these difficulties by organizing a second field expedition, this one at roughly the same season as the fateful spillover that killed Emile Ouamouno, but a year later and in neighboring Ivory Coast. Angolan free-tailed bats are abundant there too, roosting beneath the roofs of village houses. Their very abundance in such close proximity to people suggests a further perplexing question, if the little-bat hypothesis is correct: With the virus so near, why don’t spillovers occur far more often? Leendertz wanted to trap those bats, as many as possible, and sample them for evidence of Ebola. Photographer Pete Muller and I went with him.
Leendertz and his team, including a graduate student named Ariane Düx, focused on two villages outside the city of Bouaké, a trade hub near the country’s center. After shopping for trap materials in Bouaké’s market, scouting the villages for bat-filled houses, and paying respects to village elders, the team assembled their apparatus late one afternoon, in time for the fly out at dusk. The traps were cone-shaped structures, jerry-built of long boards and translucent plastic sheeting, designed to capture bats as they emerged from a roof hole and funnel them down into a plastic tub. Amazingly, the system worked. At 6:25 p.m. on the first evening one trap came alive like a popcorn popper, as dozens of small gray bodies slid down the sheeting and thumped into the tub.
For the next phase Leendertz and Düx suited up in medical gloves, respirator masks, gowns, and visors. With a naked lightbulb hanging above their makeshift lab table, they began processing bats: weighing and measuring each animal, noting sex and approximate age, injecting an electronic chip the size of a caraway seed for later identification, and most important, drawing blood from a vein in the animal’s tiny arm. One well-aimed poke with a delicate needle, and a blood drop would appear, to be gathered with a fine pipette. Düx and Leendertz worked together at close range, trustingly sharing tasks, and it occurred to me that if she poked twice at the vein and missed the second time, jabbing Leendertz’s finger instead, he could have an Ebola-related needle-stick injury. But she didn’t miss.
The blood went into small vials, for freezing immediately in a liquid-nitrogen tank and eventual screening back in Berlin. A small fraction of all the captured bats would be killed and dissected, so that snippets of their internal organs, especially liver and spleen, where viruses often concentrate, could be added to the trove of frozen samples. The other bats would be released. If a blood sample from one dissected individual later tested positive for antibodies or viral fragments, its organs would then be used in an attempt (more dangerous and more expensive, done only in a BSL-4 laboratory) to isolate live Ebola virus.
After a few bats Leendertz stepped back from the processing work and allowed an Ivorian graduate student, Leonce Kouadio, tall, mild mannered, and thin as a candle, to take his place. This was a training mission as well as a scientific investigation, after all, and Leendertz wanted to give his protégés a richness of experience. Kouadio had good skills already, and as he got into rhythm, sharing these exacting tasks in the warm African night, I noticed the T-shirt beneath his medical gown, which carried some sort of resort logo and said, It’s the perfect holiday. For him, maybe, but not for everybody.
A Strange Host
Back in the United States, I spoke with more experts during a stop at the CDC in Atlanta and by telephone. When I asked why it’s important to identify the reservoir host of Ebola virus, they all agreed: because that information is essential to preventing future outbreaks. On other points they diverged. The most unexpected comment came from Jens Kuhn, a brainy young virologist now at the National Institutes of Health and, by way of his tome Filoviruses, arguably the preeminent historian of Ebola. I’ve known Kuhn as a candid source but also a lively and generous friend since we met at a conference hosted by Eric Leroy. Why do you think that after 39 years, I asked him, the reservoir of Ebola is still unidentified?
“It’s a strange host.”
“A strange host,” I repeated, not sure I’d heard right.
“That’s what I think.”
His logic was complex, but he sketched it concisely. First, outbreaks of Ebola virus disease have been relatively infrequent—only about two dozen in nearly 40 years. Rare occurrences. Almost everyone was traceable to a single human case, infected from the wild, followed by human-to-human transmission. This suggests, he said, that the sequence of events yielding spillover has to be “extraordinary and weird.” Highly unusual circumstances, an unlikely convergence of factors. Second, there’s “the remarkable genome stability of the virus over the years.” It didn’t change much, didn’t evolve much, at least until the human case count in West Africa started going so high, providing many more opportunities for the virus to mutate. That stability might reflect “a bottleneck somewhere,” Kuhn said—a constraining situation that keeps the virus scarce and its genetic diversity low. One possible form of bottleneck would be a two-host system: a mammal host such as a bat species that becomes infected only intermittently, when it gets bitten by a certain insect or tick or other arthropod, perhaps relatively rare or narrowly distributed, which is the ultimate host of the virus. As we both knew, this harked back to that hitchhiker in Rhodesia in 1975 who suffered an odd little bite and then died of Marburg. It evoked the spider in Bob Swanepoel’s lab that carried Ebola for two weeks.
What would you do, I asked him, if you had a big research grant for nothing but finding Ebola’s reservoir? Kuhn laughed.
“I’m going to make myself unpopular,” he said, “but I would still look into insects and other arthropods.”
He doesn’t have that big grant, nor does anyone else. The mystery remains. The stakes are high. The samples from Ivory Coast have so far yielded no positives. The search continues.
Originally published by National Geographic Magazine and natgeo.com in July 2015.
Credits
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Director
Author
Production Managers
Program Specialists
Producer
other
Last Updated
September 4, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
Media
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text
Text on this page is printable and can be used according to our Terms of Service.
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.